Abstract
Background
The 2022 KDIGO guidelines now recommend the initiation of sodium glucose-cotransporter 2 inhibitors (SGLT2i) in patients with chronic kidney disease (CKD) and type-II diabetes (T2D) with an eGFR ≥ 20 mL/min/1.73m^2 to slow the progression of kidney dysfunction. Despite updated standard of care recommendations, the uptake of SGLT2is in eligible patients is suboptimal.
Objective
This research aimed to observe the prescribing patterns of SGLT2is by primary care resident physicians in eligible patients with T2D and CKD G3a/3b.
Methods
This study utilized a data analytics platform to identify adults with CKD G3a/G3b and T2D at two primary care clinics, comprised of more than 100 resident physicians between July 1st, 2023, and October 31st, 2023. The primary objective of this study was to identify the proportion of patients currently prescribed an SGLT2i. Secondary objectives included identifying the proportion of patients: (1) never prescribed an SGLT2i, (2) previously prescribed an SGLT2i but not currently on therapy, and (3) not currently on SGLT2i therapy but may qualify for initiation based on current clinical practice guidelines
Results
A total of 240 patients were identified with CKD G3a/G3b and T2D. 111/240 (46.2%) were removed from the primary care resident cohort due to eGFR values falling outside the CKD G3a/G3b criteria at the time of review. 12/240 (5%) were not observed as they have not seen their primary care resident physician in more than 12 months. 46/240 (19.2%) were observed in the specialist cohort as they were managed by endocrinology or nephrology. This left 71/240 (29.6%) patients observed in the primary care resident physician cohort for SGT2i use. Among these, 27/71 (38%) were currently prescribed an SGLT2i, while 44/71 (62%) were identified as potential candidates for therapy initiation.
Conclusion
This analysis describes the underutilization of SGLT2is in eligible patients, despite updated standard of care recommendations and evidence to support its benefit.
Introduction
Chronic kidney disease (CKD), defined as abnormalities of kidney structure or function, affects nearly 800 million patients globally [1,2]. CKD is classified based on Cause, Glomerular filtration rate (GFR) category (G1-G5), and Albuminuria category (A1-A3), abbreviated as CGA [1]. Criteria include either markers of kidney damage (albuminuria ≥ 30 mg/g, abnormal urine sediment, imaging or histology findings, electrolyte disorders, or kidney transplant history) or GFR < 60 mL/min/1.73m^2 [1]. CKD increases the risk of dialysis or transplantation, cardiovascular disease, and premature death [3]. T2D is the leading cause of CKD and is recognized as the most significant risk factor, with nearly 40% of patients developing diabetic kidney disease (DKD) [4]. Before sodium-glucose cotransporter 2 inhibitors (SGLT2is), treatment focused on blood pressure control with angiotensin converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs), which reduced proteinuria but not mortality or cardiovascular events in major CKD trials for patients with co-morbid T2D [4].
Recent landmark trials, including CREDENCE, DAPA-CKD, and EMPA-KIDNEY, have demonstrated that SGLT2is slow the progression of CKD in patients with T2D by reducing glomerular hyperfiltration and tubuloglomerular feedback [5-8]. Additionally, canagliflozin, empagliflozin, and dapagliflozin reduced the risk of death from cardiovascular events, hospitalizations for heart failure, and death from any cause in patients with and without diabetes. In response to the proven cardiorenal benefits of SGLT2is in these trials, the KDIGO guidelines now recommend the initiation of SGLT2is in patients with T2D and CKD with an eGFR ≥ 20 mL/min/1.73m^2, with continuation as tolerated, until dialysis is initiated, or transplantation is completed [9].
Despite updated standard of care recommendations, SGLT2is remain underutilized in eligible patients. In 2021, a cross-sectional study at Mass General Brigham Center showed only 6% (n = 22,653) of patients with CKD G3-G5 and T2D were currently prescribed an SGLT2i [10]. A national sample of U.S. veterans with T2D, CKD, and cardiovascular disease was studied from January to December 2023. As a result, only 11.5% (n = 174,433) of patients were currently prescribed an SGLT2i [11]. In Seoul, 3,703 patients were identified with T2D and CKD. Of which, only 32.9% were prescribed an SGLT2i, and the presence of congestive heart failure (CHF) or coronary artery disease (CAD) significantly increased the odds of SGLT2i initiation [12]. While these studies consistently demonstrate a gap between guideline recommendations and prescribing patterns, they do not distinguish prescribing patterns by provider type; that is, between attending vs. resident physicians, or between primary and specialist prescribers. This study, therefore, specifically examines SGLT2i prescribing among eligible empaneled patients managed by primary care resident physicians.
Methods
Study Design and Setting
This was a retrospective, observational, cohort-based, quality improvement study at two general internal medicine clinics, comprised of more than 100 medical residents as part of a large academic medical center in the Midwest. The first phase of this study included a primary care resident physician utilizing a third party, health insurance portability and accountability act (HIPAA) protected, data analytics tool to identify and diagnose patients with CKD G3a/G3b in accordance with standard of care, defined as an eGFR of 30-60 mL/min/1.73m^2 between July 1st and October 31st, 2023, as part of a resident-led quality improvement project. The second phase of this study occurred between July 1st and October 31st, 2024, where an embedded primary care pharmacist utilized the customized data analytics report to cross-reference the newly diagnosed population for concomitant T2D diagnoses to identify the patients with both CKD and T2D for this study.
Data Analytics Platform
This study leveraged Qlik®, a proprietary business intelligence and data analytics platform, to streamline retrospective chart reviews in the electronic health record (EHR). Qlik® is a web-based platform that integrates data from multiple sources and enables the creation of interactive reports and dashboards for visualization and analysis. For this study, key data points were embedded into the customized report, including patient demographics (age, race, insurance), current medications, discontinued medications, and the most recent collection of urine albumin creatinine ratio (uACR), hemoglobin A1c (HbA1c), and eGFR. Rather than relying on manual chart review, the platform automatically extracted these data points, reducing effort and improving efficiency for the research team. The data was then visualized in a dashboard through interactive tables and graphs, facilitating early identification of trends and insights. For example, the tool generated a table stratifying patients by CKD category, enabling visualization of those whose eGFR fell outside the CKD G3a/G3b range at analysis. It also produced a pie chart illustrating insurance type distribution (Medicare, Medicaid, commercial, or self-pay) and graphs displaying average serum creatinine and HbA1c. From these interactive visualizations, individual patients were included for detailed review by the research team. The project creation and timeline are shown in Figure 1, illustrating the customizations made by the research team and the timeliness of data collection.
Population
Adults 18 years or older with T2D and CKD who were empaneled to a primary care resident physician from July 1st, 2023, to October 31st, 2023, were included in this study, as part of the primary care resident physician cohort. If patients were actively following with nephrology or endocrinology for CKD or T2D management (defined as a visit within the last 12 months), they were placed in the specialist cohort. Patients were not observed if they had not been seen by a primary care resident physician at either resident practice location in more than 12 months, had a diagnosis of type-I diabetes (T1D), were undergoing dialysis, or had a history of a kidney transplant. Additionally, patients were not observed if their most recent eGFR fell outside the CKD G3a/G3b criteria, as this was the initial patient population identified by the primary care resident physician during the first phase of the study.
Objectives
The primary objective of this study was to identify the proportion of patients with T2D and CKD receiving an SGLT2i at two outpatient medical resident clinics. The secondary objective was to identify the proportion of patients with T2D and CKD (1) never prescribed an SGLT2i, (2) previously prescribed an SGLT2i but not currently on therapy, and (3) not currently on SGLT2i therapy but may qualify for initiation based on current clinical practice guidelines.
Data Collection
The data analytics tool collected the following information for each patient: demographics, current and previous use of SGLT2is or ACEi/ARBs, uACR, HbA1c, and eGFR. The clinical pharmacist utilized this collected data within the report to identify the patients currently on SGLT2i. Retrospective chart review in the EHR was manually used to collect the following data: primary care provider name, whether the patient followed with endocrinology or nephrology, and clarifying information regarding previous use of SGLT2is and the reason for discontinuation.
Statistical Methods
Collected data was analyzed using descriptive statistics to determine the incidence of SGLT2i prescribing by resident physicians in patients with T2D and CKD. Baseline characteristics are presented as mean and standard deviation for continuous variables or frequency and proportion for categorical variables. Primary and secondary objectives are presented as frequency and proportion. Descriptive statistics were performed in Microsoft Excel (version 16.100.1, Microsoft Corporation, Redmond, WA, USA). Baseline characteristics are not presented for patients in the specialist cohort, as this was not a primary or secondary objective of the study.
Results
Between July 1st and October 31st, 2023, 524 patients were identified and diagnosed with CKD G3a/G3b by the primary care resident physician. Cross-referencing with T2D diagnoses identified 240 patients for the initial data analytics report. Of these, 12 patients were not included for observation as they had not been seen by their primary care resident physician within the last 12 months. Another 111 patients were not included for observation due to eGFR values falling outside the CKD G3a/G3b range at the time of review: eGFR < 20 mL/min/1.73m^2, on dialysis, or had a history of kidney transplant (n=63); eGFR 20 to < 30 mL/min/1.73m^2 (n=25); and eGFR > 60 mL/min/1.73m^2 (n=23). An additional 46 patients were managed by specialists (endocrinology or nephrology); thus, they were placed in the specialist cohort. The final primary care resident physician cohort consisted of 71 patients, with baseline characteristics presented in Table 1.
Primary and Secondary Objectives for Primary Care Resident Physician Cohort
The proportion of patients currently prescribed an SGLT2i by a primary care resident physician is shown in Figure 2. Of the 44 patients who could have benefited from SGLT2i initiation, 40/44 (90.9%) had never been prescribed an SGLT2i, and 4/44 (9.1%) had previously been on an SGLT2i but discontinued therapy due to cost (n=1) or an acute kidney injury (AKI) (n=3).
Primary and Secondary Objectives Specialist Cohort
Of the 46 patients identified with CKD G3a/G3b and T2D who were being followed by endocrinologists or nephrologists, 26/46 (56.5%) were currently prescribed an SGLT2i, while 20/46 (43.5%) were identified as potential candidates for therapy initiation. Of the 20 patients who could have benefited from SGLT2i initiation, 13/20 (65%) were never prescribed an SGLT2i, and 7/20 (35%) had previously been on an SGLT2i but discontinued therapy due to an AKI (n=2) or an adverse drug reaction (n=5).
Discussion
This report found the underutilization of SGLT2is in eligible patients at our two general internal medicine clinics among primary care resident physicians. Among eligible patients, primary care resident physicians prescribed an SGLT2i in 38% of cases. Although not comparable, eligible patients who were under the care of a specialist were prescribed an SGLT2i 56.5% of the time. Differences in specialist training should be considered when interpreting these findings. Overall, SGLT2i prescribing for patients with T2D and CKD G3a/G3b remained low across providers, both primary care resident physicians and specialists.
Despite updated guidelines and recommendations, several barriers may prevent primary care resident physicians from prescribing this medication class. A commonly cited barrier in literature by primary care physicians, though it is not always clear whether it applies to residents or attendings, is the under-appreciation for cardiorenal benefits for SGLT2is. Many general practitioners remain unaware that the cardiovascular and renal advantages of SGLT2is are independent of their glucose-lowering effects. Some physicians also express a lack of confidence due to limited understanding of the clinical outcomes associated with their use [13, 14]. Given that T2D is primarily managed in the primary care setting, ensuring that both resident physicians and attendings are well-informed on both the benefits and risks of SGLT2is is essential. Maintaining up-to-date knowledge of evolving guidelines and emerging data can be challenging, as it can take up to 17 years for novel therapies and new recommendations to be used in clinical practice [15]. However, embedded pharmacists in primary care clinics can play a critical role in providing education and support to physicians. In 2024, a multidisciplinary group of physicians and pharmacists created an initiative in New York to increase the uptake of SGLT2i prescribing. The group offered SGLT2i education sessions, practice support tools, dashboards, and patient education materials to prescribers, which resulted in a 37.4% increase in prescribing [16].
In addition, cost remains a major barrier to prescribing, affecting both primary care and specialty settings. This challenge is particularly pronounced in the United States, where health insurance plans can limit access to SGLT2is more than in countries with publicly funded healthcare systems. In a survey of nephrologists, 37% reported that lack of insurance or high medication cost hindered their prescribing [17]. Similarly, a survey of 202 physicians across internal medicine, family medicine, cardiology, endocrinology, and nephrology practices identified high out-of-pocket costs and insurance limitations as key obstacles to initiating therapy [18]. Most respondents indicated that the majority of their patients were insured by Medicare, which created additional barriers to affordable access given high deductible and co-pay costs [18]. Notably, physicians with a clinic-embedded pharmacist were less likely to report cost as a barrier, highlighting the important role pharmacists can play in supporting prescribing decisions by providing education on medication access, insurance coverage, and affordability [18]. Although this study did not specifically assess the reasons for not prescribing SGLT2is in eligible patients, it is notable that more than half were insured through Medicare. While SGLT2is are included on most Medicare formularies, the brand-name options can still be cost-prohibitive due to high deductibles and cost-sharing requirements.
Although this report highlights the underutilization of SGLTi2s in eligible patients, there are several notable limitations. For external validity, we suspect this data can be generalized to other medical resident primary care clinics, but is likely not generalizable to clinics managed by attending primary care physicians. As such, attending primary care physicians may have different levels of clinical expertise and familiarity with clinical guidelines. This study may also be subject to sampling bias, as a large sample of patients was not observed. The initial data analytics report used to identify patients for CKD G3a/G3b diagnosis did not extend below an eGFR of 30 mL/min/1.73m^2, despite the KDIGO recommendation to initiate SLGT2is with an eGFR ≥ 20 mL/min/1.73m^2. Meaning, patients with an eGFR ≥ 20 but < 30 mL/min/1.73m^2 were not identified as potential candidates. Additionally, a diagnosis of CKD G3a/G3b was based solely on eGFR and did not include the presence of albuminuria or other markers of kidney damage. The initial report utilized for diagnosis by the primary care resident physician was created more than 1 year ago, so patients more recently diagnosed with CKD G3a/G3b were also not included.
A strength of this study was the use of a data analytics tool to systematically identify patients already receiving standard of care therapy, and more importantly, those that would benefit from SGLT2i initiation. This approach enabled a more targeted review process, reducing reliance on manual chart review and allowing for streamlined evaluation of prescribing patterns. By generating a report of patients with T2D and CKD G3a/G3b that incorporated 90% of the required data points, the tool reduced the need for manual chart review by the research team. The dashboard also provided insights into baseline characteristics such as average eGFR, A1c, race, gender, and insurance type, which allowed the researchers to categorize the study populations even before data collection was complete. A second strength of this study was the use of actual prescribing data for SGLT2is by primary care resident physicians, which enhances the clinical relevance of the findings and increases the potential generalizability to other resident physician practices.
As a result of this study, 44 patients were identified as potential candidates for SGLT2i initiation. Through interdisciplinary collaboration, future directions include proactive outreach to eligible patients to initiate standard of care therapy. In parallel, a primary care resident physician will provide education to primary care resident physician colleagues on the benefits, risks, cost considerations, and safe prescribing practices of SGLT2is through presentations and informational materials, with the ultimate goal to promote appropriate SGLT2i prescribing in eligible patients through shared decision-making. By addressing under-prescribing and equipping providers with medication class knowledge, this approach may serve as a framework for guiding initiatives aimed at identifying and closing care gaps.
Conclusions
This study suggests the underutilization of SGLT2is in patients with T2D and CKD G3a/G3b within the primary care resident physician cohort, despite proven benefit in clinical trials and updated standard of care recommendations. Collaborative, interdisciplinary efforts between pharmacists and resident physicians may help address this gap by providing targeted guidance on the safe and effective implementation of updated treatment recommendations. Additionally, leveraging data analytics tools to efficiently identify care gaps allows for proactive outreach and population-level interventions, supporting timely adoption of updated clinical guidelines and improved patient outcomes.
Tables and Figures

Figure 1. Project creation and timeline.
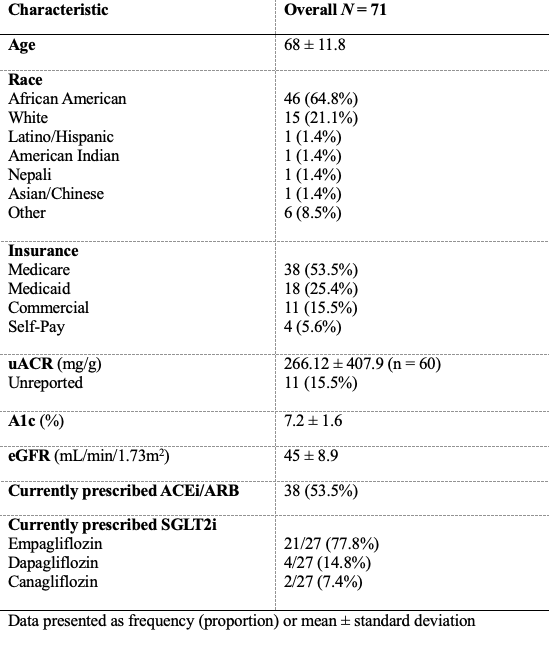
Table 1. Characteristics of SGLT2i prescribing within primary care clinics.

Figure 2. Proportion of patients with an estimated glomerular filtration rate of 30-60 mL/min/1.73m^2 prescribed an SGLT2i by a primary care resident physician (n=71).
Details
Acknowledgements
None.
Disclosures
None.
Data Availability Statement
Not applicable.
References
- 1
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(4S):S117-S314.
- 2
Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 2022;12(1):7-11.
- 3
Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294-1304.
- 4
Mark PB, Sarafidis P, Ekart R, et al. SGLT2i for evidence-based cardiorenal protection in diabetic and non-diabetic chronic kidney disease: a comprehensive review by EURECA-m and ERBP working groups of ERA. Nephrol Dial Transplant. 2023;38(11):2444-2455.
- 5
Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
- 6
Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-1446.
- 7
The EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127.
- 8
Yau K, Dharia A, Alrowiyti I, Cherney DZI. Prescribing SGLT2 inhibitors in patients with CKD: expanding indications and practical considerations. Kidney Int Rep. 2022;7(7):1463-1476.
- 9
Rossing P, Caramori ML, Chan JCN, et al. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5):S1-S127.
- 10
Zhuo M, Li J, Buckley LF, et al. Prescribing Patterns of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with CKD: A Cross-Sectional Registry Analysis. Kidney360. 2022;3(3):455-464.
- 11
Gregg LP, Ramsey DJ, Akeroyd JM, et al. Predictors, disparities, and facility-level variation: SGLT2 inhibitor prescription among US veterans with CKD. Am J Kidney Dis. 2023;82(1):53-62.e1.
- 12
Jeong, S.J., Lee, S.E., Shin, D.H. et al. Barriers to initiating SGLT2 inhibitors in diabetic kidney disease: a real-world study. BMC Nephrol. 22, 177 (2021).
- 13
Milder TY, Stocker SL, Baysari M, Day RO, Greenfield JR. Prescribing of SGLT2 inhibitors in primary care: a qualitative study of general practitioners and endocrinologists. Diabetes Res Clin Pract. 2021;180:109036.
- 14
Ng NM, Ng YS, Chu TK, Lau P. Factors affecting prescription of sodium-glucose co-transporter 2 inhibitors in patients with type 2 diabetes mellitus with established cardiovascular disease/ chronic kidney disease in Hong Kong: a qualitative study. BMC Prim Care. 2022;23(1):317.
- 15
Nishi L, Ghossein C, Srivastava A. Increasing sodium-glucose cotransporter-2 inhibitor use in CKD: perspectives and presentation of a clinical pathway. Kidney Med. 2022;4(5):100446.
- 16
Walsh A. Education improves SGLT2 inhibitor and GLP1-RA prescribing, benefits cardiology patients. Pharmacy Times. 2024 Jan 2. Available from: www.pharmacytimes.com/view/education-improves-sglt2-inhibitor-and-glp1-ra-prescribing-benefits-cardiology-patients.
- 17
Singh T, Li T, Mandelbrot D, Astor BC, Mehr AP. Prescribing Patterns for Sodium-Glucose Cotransporter 2 Inhibitors: A Survey of Nephrologists. Kidney Int Rep. 2023;8(8):1669-1671.
- 18
Thompson K, Bowers BL, Evans AM. Self-identified prescriber tendencies in sodium-glucose cotransporter-2 inhibitor outpatient prescribing. J Am Pharm Assoc (2003). 2024;64(3):102068.